Uses of sodium hydroxide
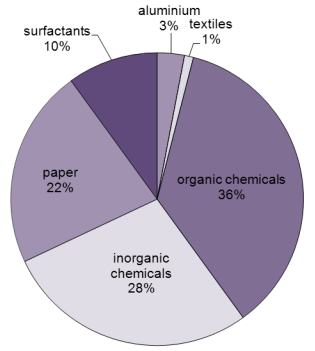
Figure 1 Uses of sodium hydroxide.
While Figure 1 shows the worldwide uses of sodium hydroxide, the proportion varies country by country. For example the maufacture of aluminium only accounts for 3% in the US whereas 35% is used to make organic chemicals, a reflection of the industrial make up of the US.
Sodium hydroxide is used in many industries (Figure 1). Much is used to scrub gases to remove acids before emitting them to the open anvironment. For example gases emitted from burning fossil fuels which contain considerable amounts of sulfur dioxide. There are many ways of trapping the sulfur dioxide, one which is used widely involves scrubbing the gases with a solution of sodium and calcium hydroxides. This technique is also used in refining processes such as the purification of bauxite (Figures 2 and 3).
|
Figure 2 Sodium hydroxide is used in the purification of the ore, bauxite, prior to it being used to make aluminium. This picture shows purified bauxite being unloaded from a ship in Iceland, on its way to an aluminium extraction plant. By kind permission of Alcoa. |
 |
Another major use of sodium hydroxide is in the manufacture of paper from wood. In the most used process, the Kraft process, wood is treated with a solution containing a mixture of sodium sulfide and sodium hydroxide. Most of the unwanted material in the wood, such as the lignins, dissolve in the liquor, leaving relatively pure cellulose which is filtered off. It is this cellulose which after further purification forms the basis of paper.
Other uses include the production of surfactants, soaps and bleaches, the latter being usually produced by passing chlorine gas into a solution of sodium hydroxide, which generates a solution containing sodium chlorate(I) (sodium hypochlorite):
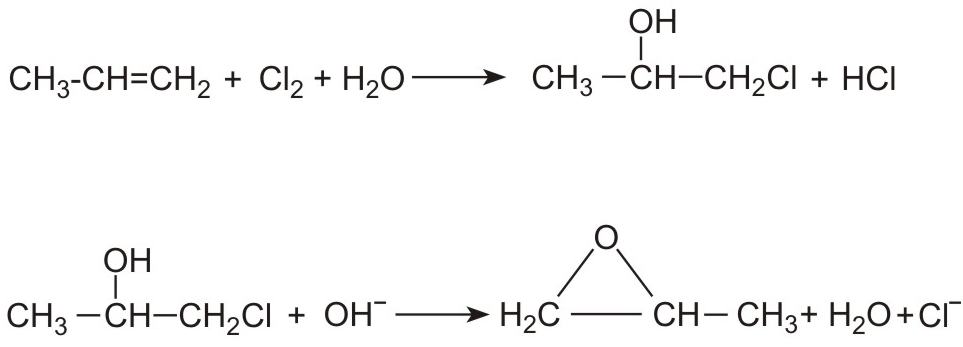
Sodium hydroxide is used in making organic chemicals, one of the most important being epoxypropane (propylene oxide), used in turn to make polyurethanes. Propene is treated with chlorine in water to make a mixture of 1-chloropropan-2-ol and 2-chloropropan-1-ol. Epoxypropane forms on addition of a solution of either sodium hydroxide or calcium hydroxide.


Annual production of sodium hydroxide
| World | 66 million tonnes |
| Europe | 18 million tonnes |
| US | 14 million tonnes |
| China | 16 million tonnes |
| Rest of Asia | 11 million tonnes |
Manufacture of sodium hydroxide
Sodium hydroxide and chlorine are manufactured together by the electrolysis of sodium chloride.
Large deposits of sodium chloride (rock salt) are found in many parts of the world. These deposits are almost pure sodium chloride and are often several hundred metres deep (some are up to 3000 metres deep) with seams of 30 m to 500 m thick (Figure 4). They were evaporated from trapped seas in the Triassic period, 200 million years ago. For example, in Europe, the seas produced deposits which stretch, although not continuously, from Cheshire, Lancashire, Staffordshire and Cleveland in the UK to Poland. They are also found throughout the US, particularly in Louisiana and Texas.
.jpg)
Figure 3 Although much rock salt is pumped to the surface as brine, some
is mined, as is being done in this large underground deposit in Cheshire.
By kind permission of the Salt Association.
A small amount is mined as rock salt, the majority is solution-mined by the controlled pumping of water at high pressure into the salt seam. A proportion of the solution-mined brine produced in this manner is evaporated to produce dry salt.
Solar salt, produced by the evaporation of sea water using solar heating, is also a source of sodium chloride.
Figure 4 Salt (sodium chloride) is being transferred to a large silo for
storage, prior to being converted to chlorine and sodium hydroxide.
By kind permission of AkzoNobel.
Saturated brine, prior to the electrolysis, is purified to precipitate calcium, magnesium and other detrimental cations by addition of sodium carbonate, sodium hydroxide and other reagents. The suspended solids are removed from the brine by settling and filtration.
There are three electrolytic processes employed today. The concentration of caustic soda produced from each of the processes varies:
- Membrane Cells: Caustic soda is produced as a pure ca 30% (w/w) solution which is normally concentrated by evaporation to a 50% (w/w) solution using steam under pressure.
- Mercury Cells: Caustic soda is produced as a pure 50% (w/w) solution, which is the concentration most commonly sold on the world market. Some is concentrated by evaporation to 75% and then heated at 750-850 K to obtain solid sodium hydroxide.
- Diaphragm Cells: The caustic soda is produced as an impure solution called 'diaphragm cell liquor' (DCL) with typical concentrations 10-12% (w/w) sodium hydroxide and 15% (w/w) sodium chloride. In order to produce the 50% (w/w) strength that is usually required, the DCL has to be concentrated using evaporation units that are much larger and more complex than those used on membrane cell plants. Large quantities of salt are precipitated during this process, which is normally reused to produce a saturated brine feed to the cells. An additional aspect of the sodium hydroxide produced in the diaphragm cell is that the product has a small amount (1%) of salt present as a contaminant, which may make the material unsuitable for some purposes.


RELATED TAGS: SODIUM HYDROXIDE PRODUCTION,WHAT IS SODIUM HYDROXIDE,USES OF SODIUM HYDROXIDE,MAKING SODIUM HYDROXIDE,WHERE TO USE SODIUM HYDROXIDE,SODIUM HYDROXIDE TO MAKE,SODIUM HYDROXIDE TO MANUFACTUR,HOW TO COMPOUND SODIUM HYDROXIDE,COMPOSITION OF SODIUM HYDROXIDE,SPESIFICATION OF SODIUM HYDROXIDE.
SOLVER CHEM

|
|

|
|

|
|




